

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355855 (N-(3-fluoro-4-((5-(3-hydroxymethyl)phenyl)pyrrolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355876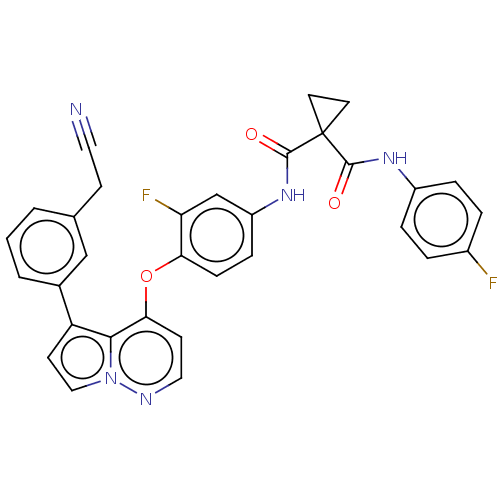 (N-(4-((5-(3-cyanomethylphenyl)pyrrolo[1,2-b]pyrida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355875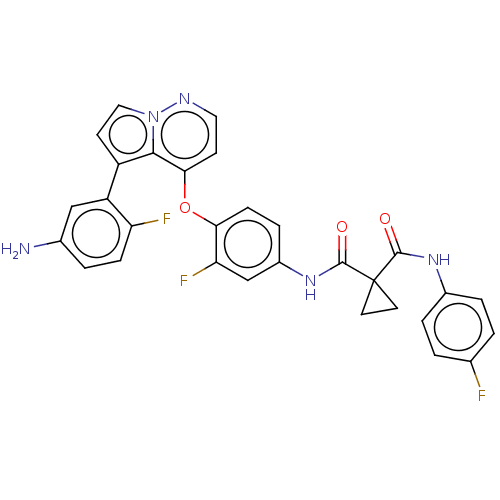 (N-(4-((5-(5-amino-2-fluoro)-5-fluorophenyl)pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355874 (N-(4-((5-(3-amino-4-methoxy)-5-fluorophenyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355873 (N-(4-((5-(3-((ethylamino)methyl)phenyl)pyrrolo[1,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355872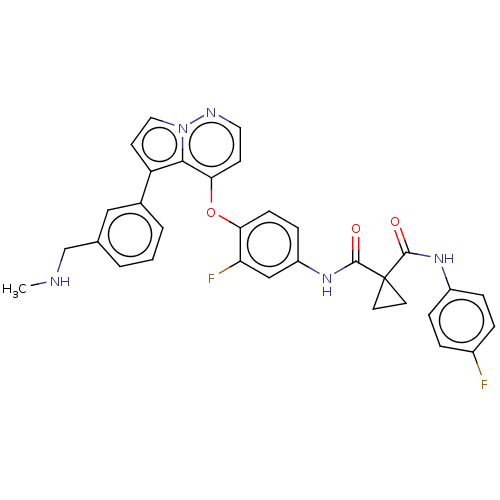 (N-(3-fluoro-4-((5-(3-((methylamino)methyl)phenyl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355871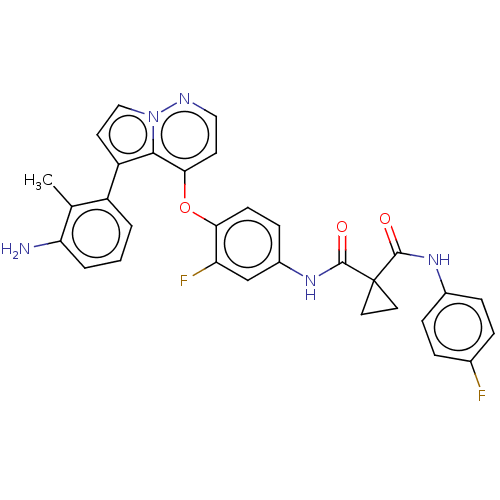 (N-(4-((5-(3-amino-2-methyl)-5-fluorophenyl)pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355870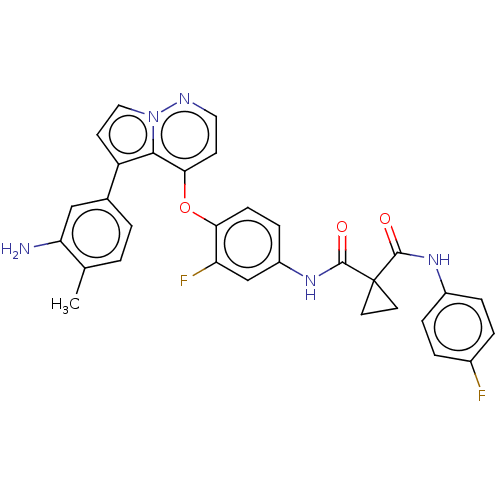 (N-(4-((5-(3-amino-4-methyl)-5-fluorophenyl)pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355868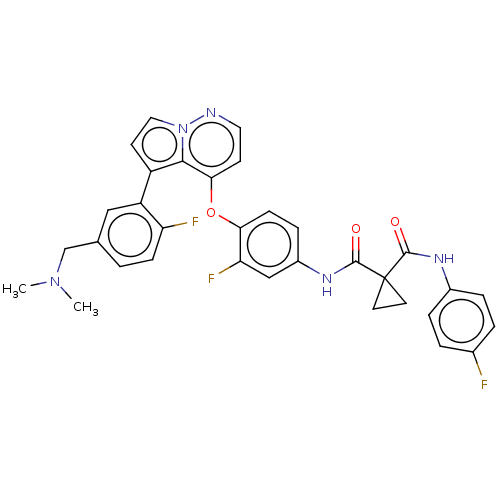 (N-(4-((5-(5-((dimethylamino)methyl)-2-fluorophenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355867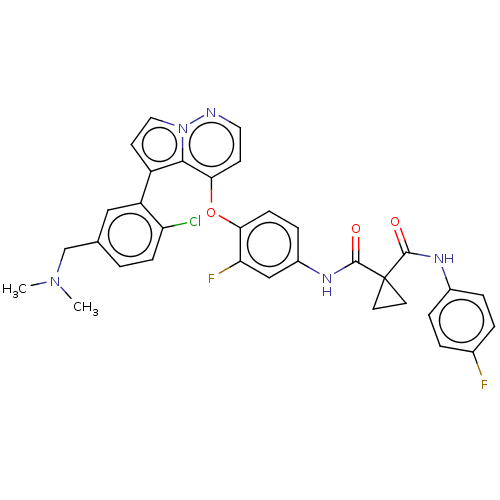 (N-(4-((5-(2-chloro-5-((dimethylamino)methyl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355866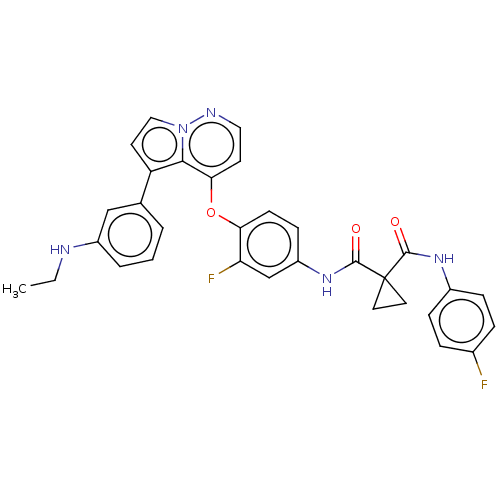 (N-(4-((5-(3-(ethylamino)phenyl)pyrrolo[1,2-b]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355865 (N-(4-((5-(3-aminomethylphenyl)pyrrolo[1,2-b]pyrida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355864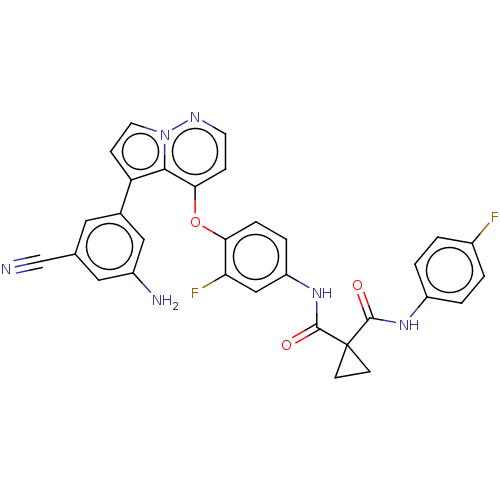 (N-(4-((5-(3-amino-5-cyanophenyl)pyrrolo[1,2-b]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355863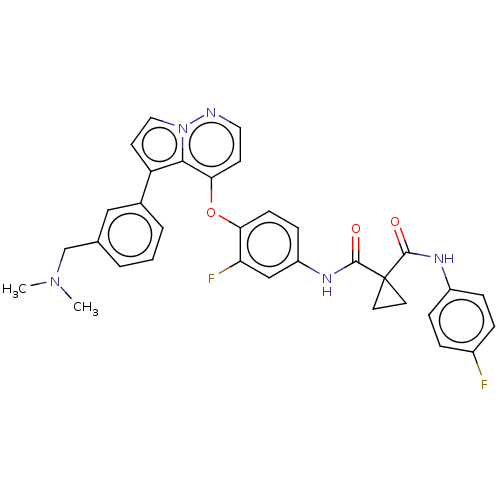 (N-(4-((5-(3-((dimethylamino)methyl)phenyl)pyrrolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355862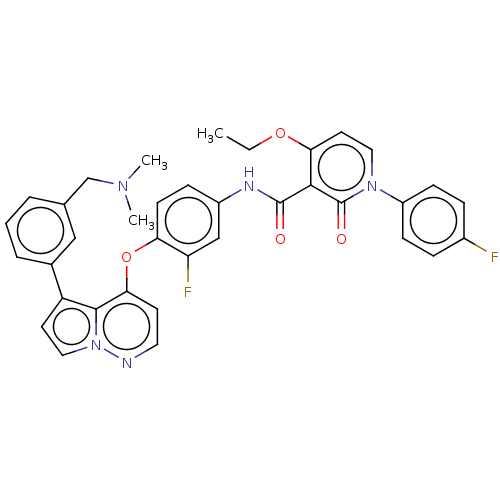 (N-(4-((5-(3-((dimethylamino)methyl)phenyl)pyrrolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355861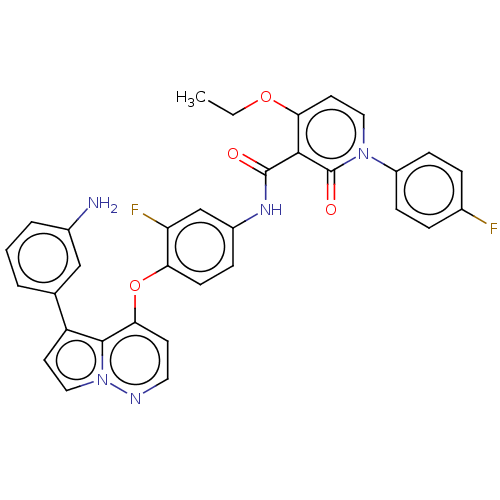 (4-ethoxy-N-(3-fluoro-4-((5-(3-aminophenyl)pyrrolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355860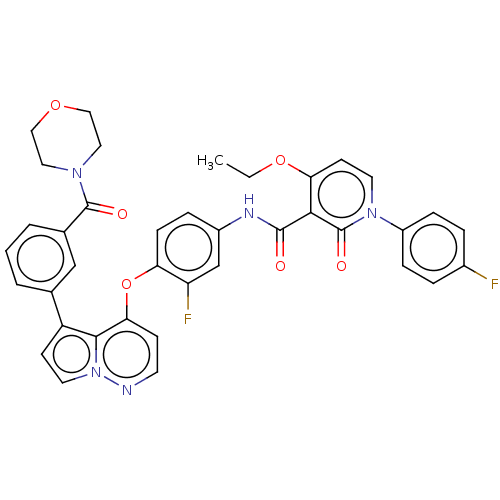 (4-ethoxy-N-(3-fluoro-4-((5-(3-morpholin-4-carbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355859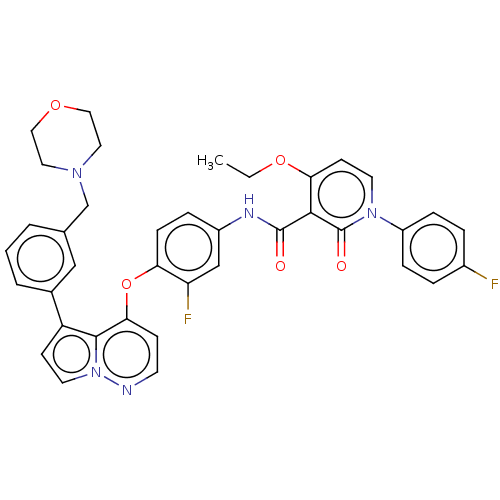 (4-ethoxy-N-(3-fluoro-4-((5-(pyrimidin-5-yl)pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355853 (4-ethoxy-N-(3-fluoro-4-((5-pyridin-4-yl)pyrrolo[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355854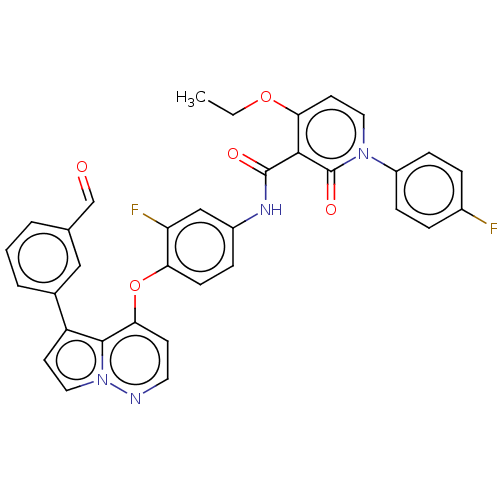 (4-ethoxy-N-(3-fluoro-4-((5-(3-formylphenyl)pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355869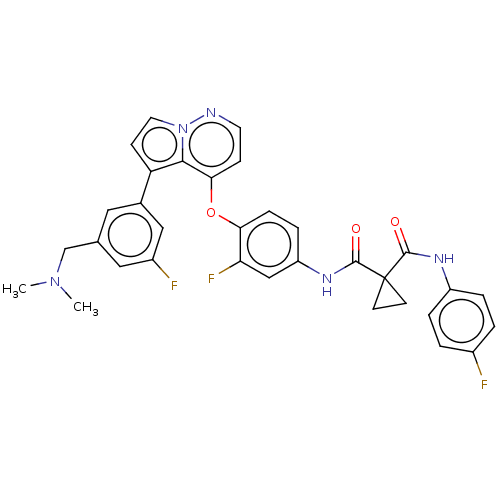 (N-(4-((5-(3-((dimethylamino)methyl)-5-fluorophenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355856 (4-ethoxy-N-(3-fluoro-4-((5-(3-(2-hydroxypropan-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355857 (4-ethoxy-N-(3-fluoro-4-((5-(3-(1-hydroxyethyl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM355858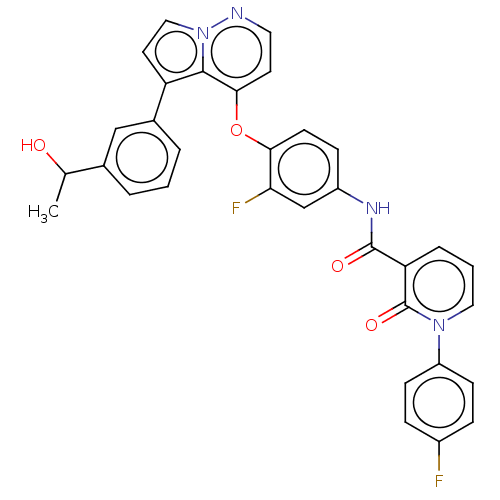 (N-(3-fluoro-4-((5-(3-(1-hydroxyethyl)phenyl)pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept... | US Patent US9815840 (2017) BindingDB Entry DOI: 10.7270/Q2862JQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117068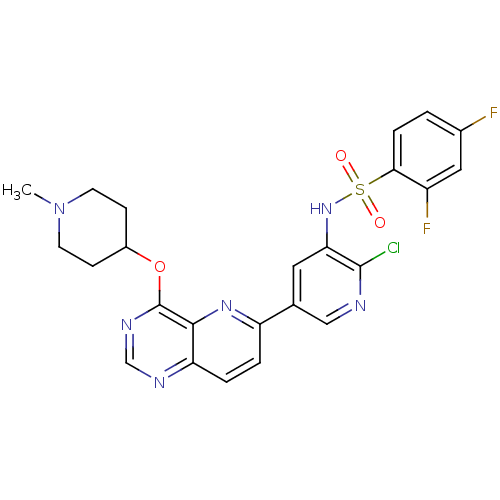 (US8664230, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117068 (US8664230, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117065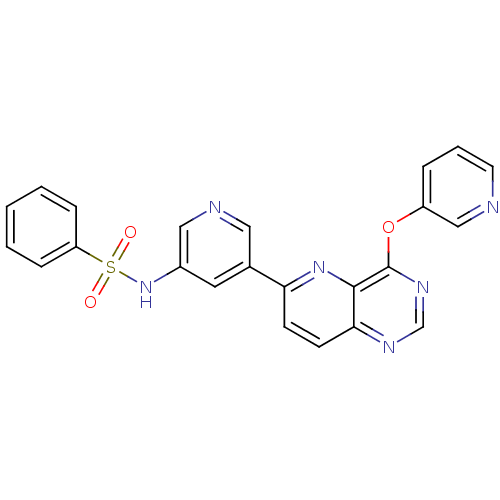 (US8664230, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117066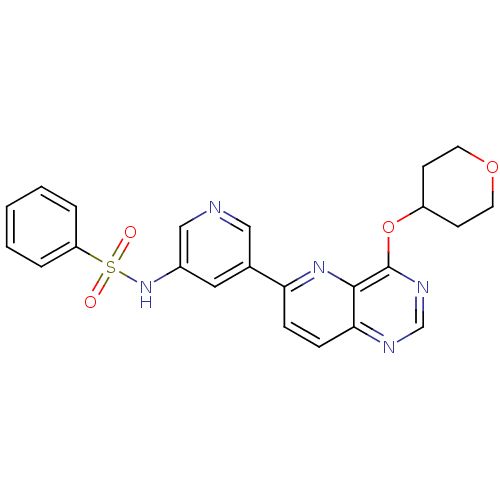 (US8664230, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117070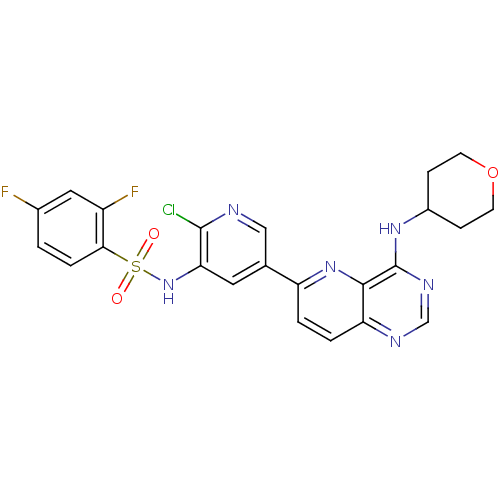 (US8664230, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117066 (US8664230, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117070 (US8664230, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117065 (US8664230, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117066 (US8664230, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117065 (US8664230, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117068 (US8664230, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117070 (US8664230, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||